38 what is the term used to label the energy levels of electrons
Energy Level ( Read ) | Chemistry | CK-12 Foundation What Are Energy Levels? Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electrons are tiny, negatively charged particles in an atom that move around the positive nucleus at the center. Energy levels are a little like the steps of a staircase. Build an Atom - Atoms | Atomic Structure | Isotope ... - PhET Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
PDF Electrons and The Structure of Atoms - Jefferson Academy Chemistry Circle the letter of the term that is used to label the energy levels of electrons. a. atomic orbitals c. quantum b. quantum mechanical numbers d. principal quantum numbers (n) 10. The letter is used to denote a spherical orbital. 11. Label each diagram below p x, p y, or p z. 12. Use the diagram above. Describe how the p x, p y, and p z ...
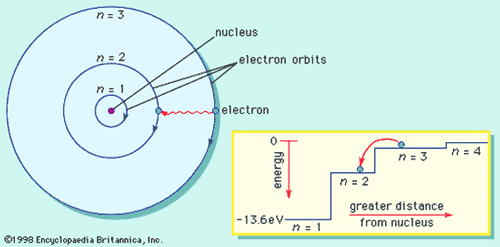
What is the term used to label the energy levels of electrons
Electrons in Atoms – Practice Worksheet IV – Learning Target 7. What term is used to label the energy levels of electrons? Principal. 8. What letter is used to denote a spherical orbital? Introduction to Weeds and Herbicides - Penn State Extension Apr 02, 2007 · In the presence of light, green plants produce sugar from carbon dioxide and water. Energy is needed for carbon, hydrogen, and oxygen atoms to rearrange and form sugar. To supply this necessary energy, electrons are borrowed from chlorophyll (the green material in leaves) and replaced by electrons split from water. How to Represent Electrons in an Energy Level Diagram You look on the periodic table and find that oxygen is atomic number 8. This number means that oxygen has 8 protons in its nucleus and 8 electrons. So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. If two electrons end up in the same orbital, one arrow faces up and the other faces down.
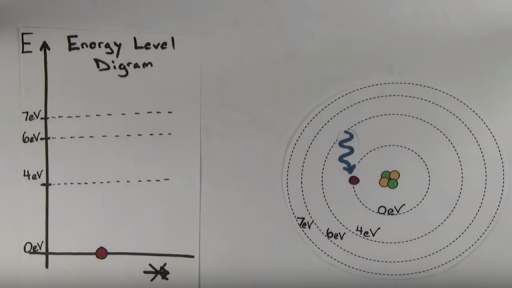
What is the term used to label the energy levels of electrons. Energy level Definition & Meaning - Merriam-Webster Definition of energy level : one of the stable states of constant energy that may be assumed by a physical system —used especially of the quantum states of electrons in atoms and of nuclei — called also energy state Examples of energy level in a Sentence Hydrogen - Wikipedia The ground state energy level of the electron in a hydrogen atom is −13.6 eV, which is equivalent to an ultraviolet photon of roughly 91 nm wavelength.. The energy levels of hydrogen can be calculated fairly accurately using the Bohr model of the atom, which conceptualizes the electron as "orbiting" the proton in analogy to the Earth's orbit of the Sun. Energy Level Diagram - Chemistry, Class 11, Structure of Atom 1) The sub shell of a particular shell do not have equal energies. For Ex: 2s and 2p have different energies. 2) In a particular shell, sub shell with lower value of l has lower energy. In the second shell, 2s ( l=0) has lower energy than 2p ( l=1) .In the 3rd shell, energies are in the order: 3s < 3p < 3d. In the 4th shells, they are in the ... Energy Level and Transition of Electrons - Brilliant According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured" by the nucleus via electrostatic forces, and impedes the freedom of the electron.
Solved An orbital energy diagram is used to show the order - Chegg An orbital energy diagram is used to show the order in which electrons are assigned to energy levels. For the diagram below, label each energy level with the correct n quantum number and the correct subshell designation, s, p ord. Question: An orbital energy diagram is used to show the order in which electrons are assigned to energy levels. Chapter 5 Chem Flashcards | Quizlet Quantum of energy is the amount of energy required to Farther the higher the electron the blank it is from the nucleus Atomic Orbital often thought of as a region of space in which there is a high probability of finding an electron Principle quantum numbers the term used to label the energy levels of electrons S used to denotate a spherical orbital Electron Energy Level Equations & Examples | What is an Energy Level of ... Each atom has different energy shells or electrons, and the shell with the highest energy is called the valence shell. Electrons contained in the valence shell are called valence electrons. Term Symbols for Atomic Energy Levels Term Symbols for Atomic Energy Levels Term Symbols The heirarchy of labels for the electrons of multi-electron atoms is configuration, term, level, and state. The term uses the multiplicity 2S + 1, total orbital angular momentum L, and total angular momentum J.
What term is used to label the energy levels of electrons? - BRAINLY The term that is used to label the energy levels of electrons are principle quantum numbers and a valance band refers to the "energy levels in an atom where the electrons that participate in bonding occupy. These energy levels correspond to those of the s and p orbitals of the outermost shell of the atom being considered." Hope this helps! :) Atomic Energy Levels - an overview | ScienceDirect Topics Atomic Energy Levels. The atomic energy level splits into multiple sublevels depending on their magnetic quantum number, which determines the component of the magnetic dipole moment of the atom projected onto the magnetic field and the Landé factor (a coefficient that relates the strength of the magnetic dipole moment to the angular momentum of an atomic energy level). Energy level - Wikipedia The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized . Tanabe–Sugano diagram - Wikipedia In a Tanabe–Sugano diagram, the ground state is used as a constant reference, in contrast to Orgel diagrams. The energy of the ground state is taken to be zero for all field strengths, and the energies of all other terms and their components are plotted with respect to the ground term.
The Periodic Table and Energy-Level Models - Middle School Chemistry An orbital defines a region within an energy level where there is a high probability of finding a pair of electrons. There can be a maximum of two electrons in each orbital. This is why the electrons are often shown in pairs within an energy level. Tell students that the rows across on the periodic table are called periods. Period 1 Hydrogen
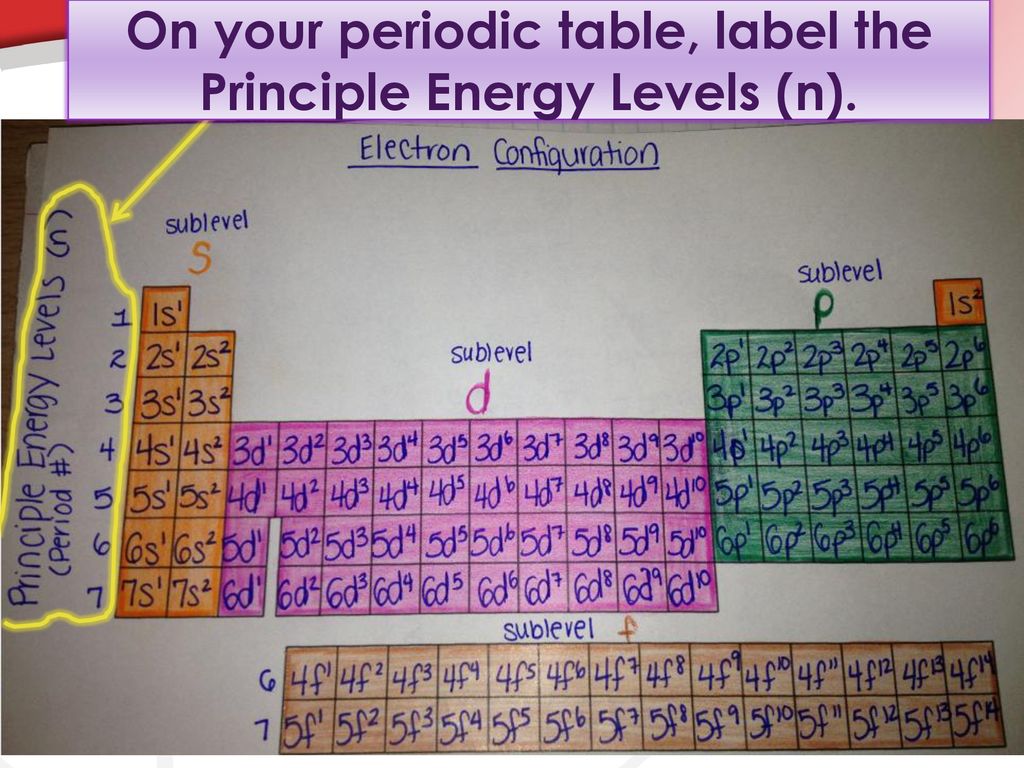
Electron Configuration Energy Levels | How to Write Electron ... The letter n denotes what energy level the electron inhabits. Any nonzero integer is a possible value for n = 1, 2, 3, and so on. This denotes the shell the electron is in. Any element in row 1...
7. What is the term used to label the energy levels | Chegg.com 7. What is the term used to label the energy levels of electrons? 8. How are s orbitals different from p orbitals? 9. How many electrons can each of the following orbitals hold? a. 2 s= 22 d. 6 d= 10 b. 3p = 146 c. 5f = 10. How many "p" orbitals can there be in any energy level? 11.
What is the term used to label the energy levels of electrons ... - Answers What term is used to label energy levels of electrons? Principal quantum numbers (n). What is the term valance band? It refers to the energy levels in an atom where the electrons that participate...
Electron Energy Level - an overview | ScienceDirect Topics Photoelectron spectroscopy is a technique whereby electrons directly ejected from the surface region of a solid by incident photons are energy analyzed and the spectrum is then related to the electron energy levels of the system.
Atomic Energy Levels (video) | Khan Academy Video transcript. - [Instructor] Here's a very simplified model of an atom. The nucleus at the center of the atom is where the protons and neutrons live, but they're kind of boring, because for the most part they just sit there. The real star of the show is the electron. The electron gets to do all the interesting stuff, like move around, jump ...
Chapter 5 Electrons in Atoms Flashcards | Quizlet What are the fixed energies of electrons called? energy levels Circle the letter of the term that completes the sentence correctly. A quantum of energy is the amount of energy required to a. place an electron in an energy level. b. maintain an electron in its present energy level. c. move an electron from its present energy level to a higher one. C
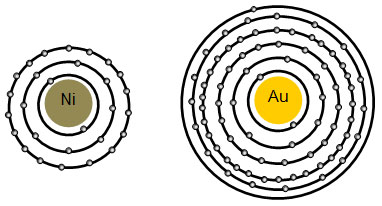
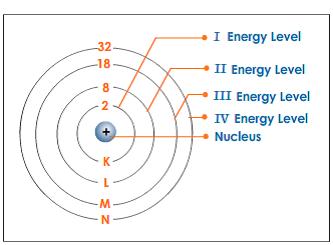
How Many Electrons Can Each Energy Level Hold? - Reference.com The maximum number of electrons that an energy level can hold is determined from the formula 2n^2 equals the total number, where n is the energy level. Thus, the first energy level holds 2 * 1^2 = 2 electrons, while the second holds 2 * 2^2 = 8 electrons. Following the formula, the third energy level can contain 18 electrons, the fourth energy ...
Clene Energy: Nanocrystal Developer Pursues Remyelination vs ... Sep 27, 2022 · Under a 42-month, up-to-$30 million term loan with Avenue entered into last year, Clene must maintain unrestricted cash and cash equivalents of at least $5 million to avoid acceleration of the ...
What term is used to label the energy levels of electrons ... - Answers What term is used to label energy levels of electrons? Principal quantum numbers (n). What is the term valance band? It refers to the energy levels in an atom where the electrons that participate...
Definition of Principal Energy Level - ThoughtCo 14 Jul 2019 — The notation used to indicate the type of energy level and the number of electrons in that level has a coefficient for the number of the ...
PDF Chemistry of Matter - Science Spot 1. Draw five protons in the nucleus of the atom. Label them with their charge. 2. Draw six neutrons in the nucleus of the atom. 3. Draw two electrons in the first energy level and label them with their charge. 4. Draw three electrons in the second energy level and label them with their charge. 5. What element is represented by the diagram?
Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS Electrons of an atom occupying particular orbitals have a particular energy. This is called energy level. When an electron alleviates from a high energy state to a lower energy state, emission of light occurs. What is an electron shell? The space around the nucleus which is filled will force more electrons out from the nucleus.
atom - Orbits and energy levels | Britannica In the same way, if energy is added to an atom, an electron can use that energy to make a quantum leap from a lower to a higher orbit. This energy can be supplied in many ways. One common way is for the atom to absorb a photon of just the right frequency.For example, when white light is shone on an atom, it selectively absorbs those frequencies corresponding to the energy differences between ...
Semiconductor moiré materials | Nature Nanotechnology Jul 14, 2022 · Electrons in moiré materials can ... (bottom). The large and small dots label the transition metal atom (where M is Mo and W) and the chalcogen atom (where X is S, Se and Te), respectively ...
Energy Level - Principal Quantum Number | Bohr's Atomic Model - BYJUS Different orbits in which electrons revolve are called energy levels or stationary states. These stationary energy/state levels for an electron are denoted as n= 1, 2, 3….. These numbers are also called the principal quantum numbers. Test your knowledge on Energy level Put your understanding of this concept to test by answering a few MCQs.
Energy Level of an Atom - Energy State and Energy level Diagrams - VEDANTU The energy levels are also called electron shells. An electron can move in one energy level or to another energy level, but it can not stay in between two energy levels. (Image will be uploaded soon) The figure shows the energy levels of an atom. The first four energy levels are shown here. The first energy level is also called level 'K'.
How to Represent Electrons in an Energy Level Diagram You look on the periodic table and find that oxygen is atomic number 8. This number means that oxygen has 8 protons in its nucleus and 8 electrons. So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. If two electrons end up in the same orbital, one arrow faces up and the other faces down.
Introduction to Weeds and Herbicides - Penn State Extension Apr 02, 2007 · In the presence of light, green plants produce sugar from carbon dioxide and water. Energy is needed for carbon, hydrogen, and oxygen atoms to rearrange and form sugar. To supply this necessary energy, electrons are borrowed from chlorophyll (the green material in leaves) and replaced by electrons split from water.
Electrons in Atoms – Practice Worksheet IV – Learning Target 7. What term is used to label the energy levels of electrons? Principal. 8. What letter is used to denote a spherical orbital?





















Komentar
Posting Komentar