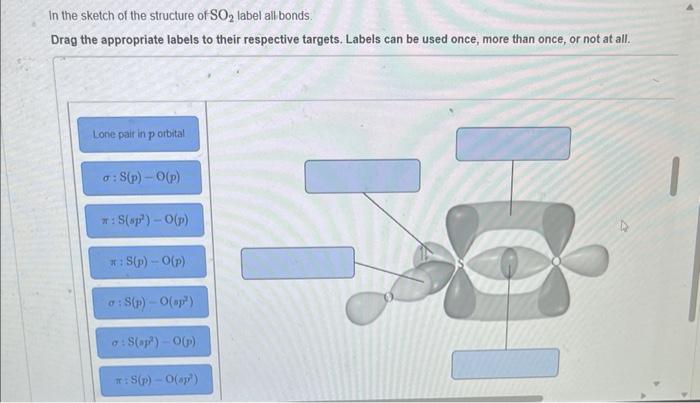
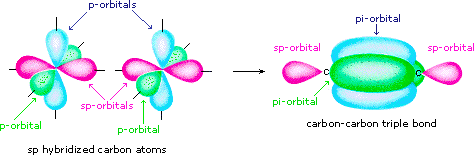
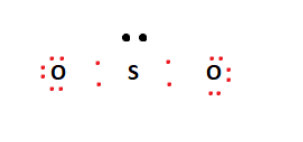
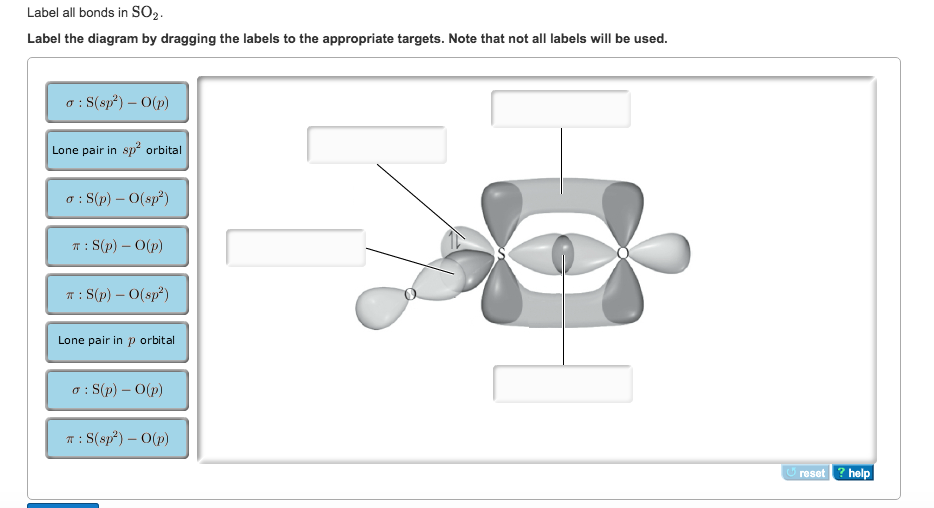
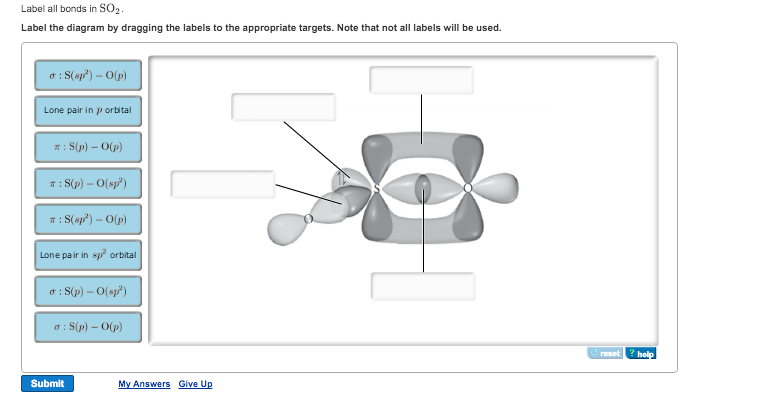
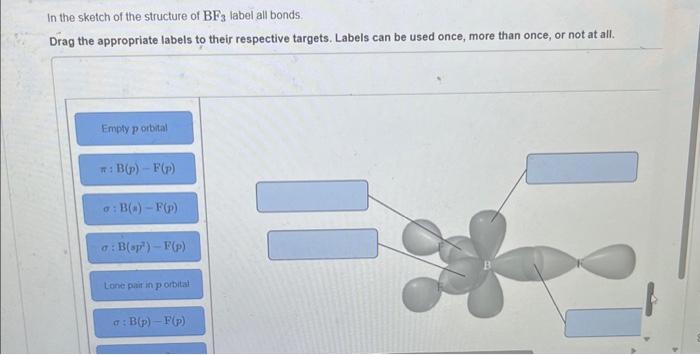
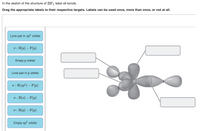
44 label all bonds in so2 .
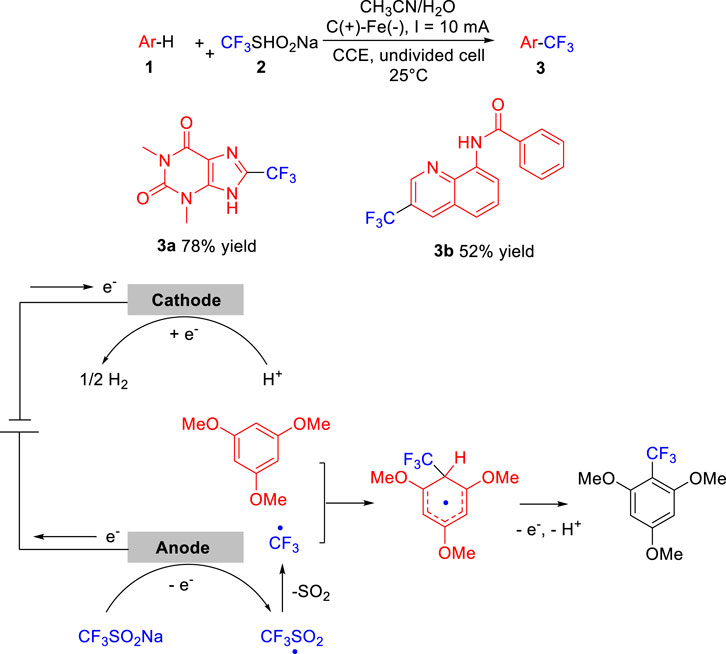
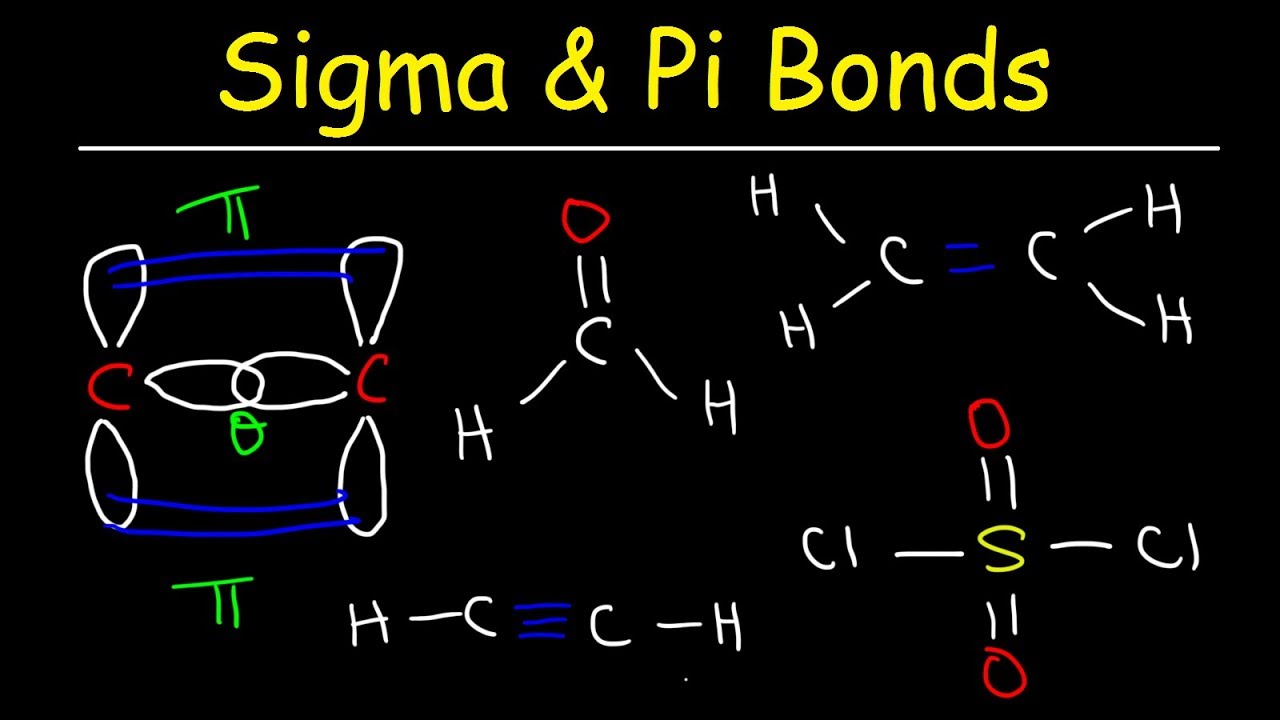
SO2 Lewis Structure, Hybridization, Molecular Geometry, and ... Before directly jumping into the lewis structure of SO2, let’s have a quick discussion regarding the importance of lewis structure and the steps to draw it. Lewis structure is the distribution of the electrons around the atoms of a compound. This structure helps us to know about the kind of bonds and the number of bonds that form the compound. Now ... [Solved] Label all bonds in SO2. Label all bonds i | SolutionInn Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Transcribed Image Text: Label all bonds in CH₂ Br2. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. a: C (sp³) - H (s) σ: C (sp³) - Br (s) ㅠ: T: C (p) - H (p) TT: C (p) - Br (p) T: C (sp³) - H (p) σ: C ...
Solved Label all bonds in SO2. Label the diagram by dragging ... Label all bonds in SO2. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. a S (sp) -O (p) Lone pair in p orbital S (p) O (p) S (spa) O (p) Lone pair in sp orbital Submit My Answers Give U This problem has been solved!

Label all bonds in so2 .
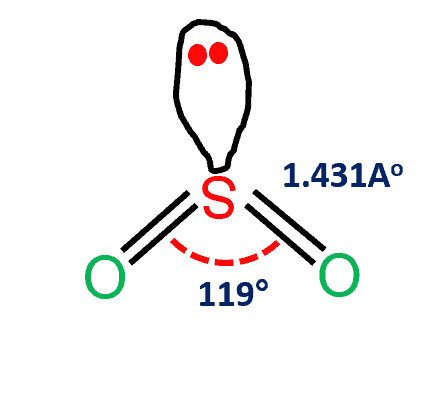
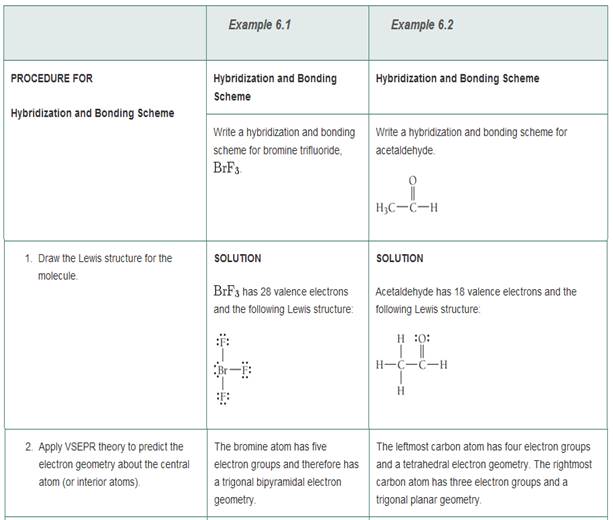
Sulfor dioxide: Lewis dot structure for SO2 (video) | Khan ... The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. CHEM: Chapter 10 Flashcards | Quizlet The sp3 and sp3d2 hybridization schemes have no unhybridized p-orbitals left to form π-bonds. Valence Bond Theory 62. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3
Label all bonds in so2 .. CHEM: Chapter 10 Flashcards | Quizlet The sp3 and sp3d2 hybridization schemes have no unhybridized p-orbitals left to form π-bonds. Valence Bond Theory 62. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3 Sulfor dioxide: Lewis dot structure for SO2 (video) | Khan ... The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen.






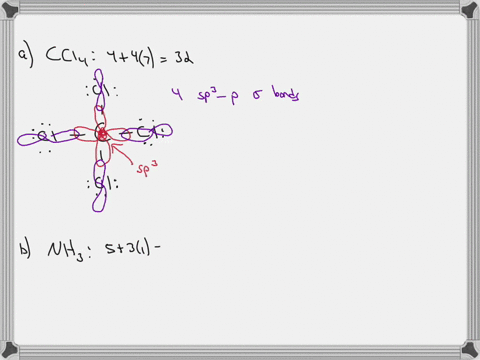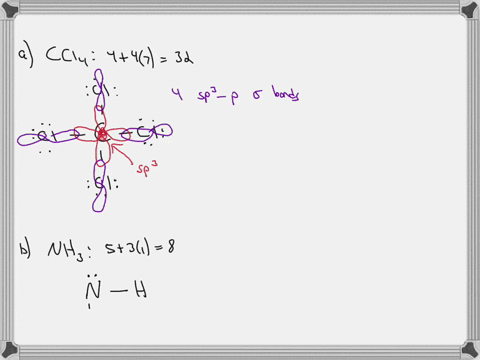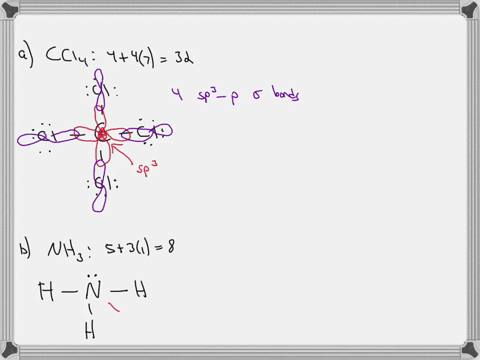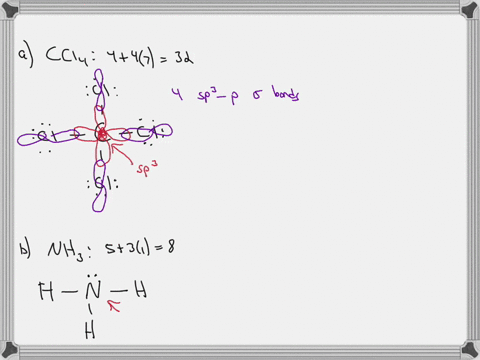








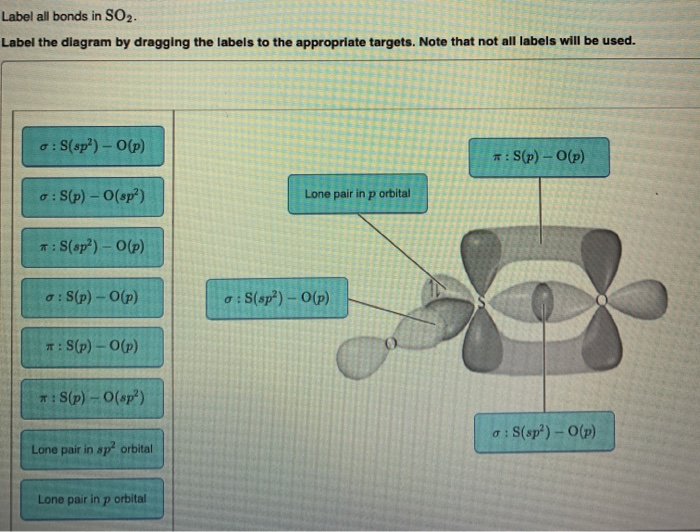




Komentar
Posting Komentar